- Atomic Mass Of First 20 Elements With Symbols
- The Elements, Sorted By Atomic Mass - Angelo State University
- All Elements Atomic Mass
- Atomic Mass Of The First 20 Elements
- - Atomic Number
- First 20 Elements Of Periodic Table
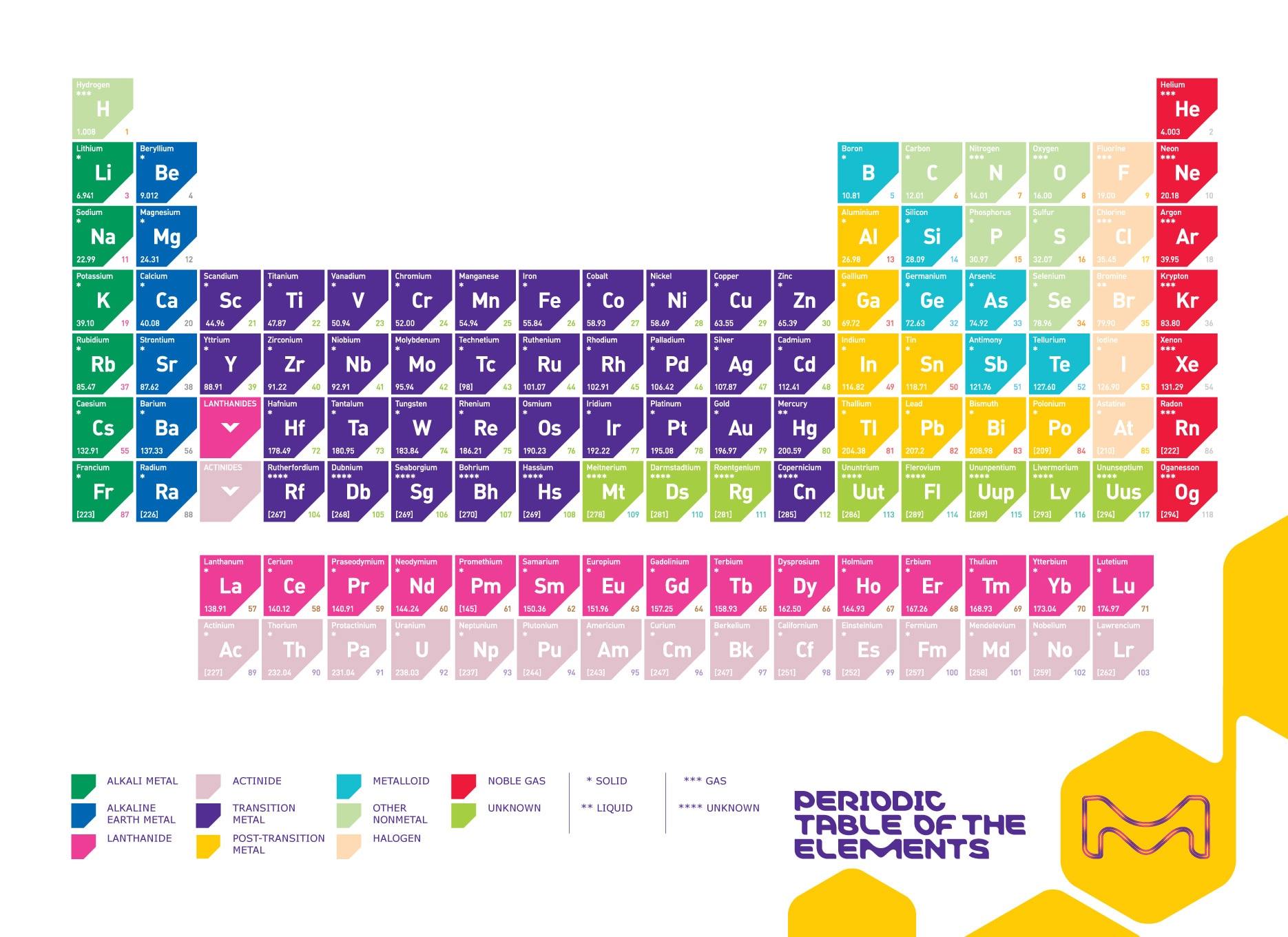
Science students in high school and colleges often find it a bit difficult to memorize the first 20 elements of the periodic table and this has posed some challenge in school especially during examinations or some kind of academic work that involves the use of any of the 20 elements of the periodic table either in determining their atomic number, mass number or reactivity.
These first 20 elements of the periodic table run across all the 8 groups in the table starting from the group on element down to the group eight elements or what is generally known as the inert gases or rare gases. As students of chemistry, understanding the chemical behavior, properties and reactions of the first twenty elements of the periodic table is the first step in having an authoritative knowledge of chemistry and its applications in all of its branches.
It is very pertinent that every science student at least at the ordinary level stage understand how the elements in the periodic table are groped and arranged in periods and groups and what properties inform the groupings of these elements. I have since learned that importance of the first 20 elements in scientific study and exploits and have devised a methodology of memorizing these first 20 elements without must task to my brain power!
Atomic mass is a characteristic of an atom that is a measure of its size. It is plays a major role in the chemical properties of elements. This article discusses atomic mass and how it is calculated. This is a list of chemical elements, sorted by atomic mass (or most stable isotope) and color coded according to type of element.Each element's atomic number, name, element symbol, and group and period numbers on the periodic table are given. The number in parenthesis gives the uncertainty in the 'concise notation' dis given in parenthesis next to the least significant digits to which it.
- This is a list of chemical elements, sorted by atomic mass (or most stable isotope) and color coded according to type of element. Each element's atomic number, name, element symbol, and group and period numbers on the periodic table are given. The number in parenthesis gives the uncertainty in the 'concise notation' dis given in parenthesis next to the least significant digits to which it.
- The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams.
- Mar 31, 2021 Can you state the atomic number and mass number of first 20 elements of P.T.? If you are confused, these flashcards can help you to understand better. While the mass quantity is the sum of the protons and neutrons in an atom, the atomic number is the value found associated with an element on the periodic table.
Now here we go…
He(H)
Has(He)
Light(Li)
Brain(Be)
But(Bo)
Could(C)
Not(N)
Offer(O)
Full(Fl)
Nine(Ne)
Subjects(Sodium, NA).
Many(Mg)

Art(Al)
Students(Si)
Pose(P)
Some(S)
Complain(Cl)
About(Ar)


Passing(potassium, K)
Chemistry(Ca)
Brief history of the periodic table.
Discovery of elements by chemists ushered in another need for studying and learning in Chemistry.
There are about 106 Elements that are known and each element discovered has to be studied.
Elements discovered have wide range of physical and chemical properties. Many are found to behave in similar ways. To make their studies convenient and orderly, chemists began to think about how they could be arranged such that the trend of behavior could be preserved among elements to have a wide range of similar physical and chemical properties.
First 20 Elements of the Periodic Table:
1. Hydrogen – H:
Symbol H. A colorless, odorless gaseous chemical element. It is the lightest and the most abundant element in the universe. It is present in water and in all organic compounds.
2. Helium – He:
Symbol He. A colorless, odorless gaseous non – metallic element belonging to group 18 of the periodic table. Helium has the lowest boiling point of all substances and can be solidified only under pressure.
3. Lithium – Li:
Symbol Li. A soft silvery metal, the first member of group 1 of the periodic table. It is a rare element found in spodumene, petalite, the mica lepidolite and certain brines. It is usually extracted by treatment with sulfuric acid to give sulphate, which is converted to the chloride. This is mixed with a small amount of potassium chloride, melted, and electrolysed.
4. Beryllium – Be:
Symbol Be. A grey metallic element of group 2 of the periodic table. It is used to manufacture Be-Cu alloys, which are used in nuclear reactors as reflectors and moderators because of their low absorption cross section.
5. Boron – B:
Symbol B. An element of the group 13 of the periodic table. It forms two allotropes, amorphous and metallic boron. The metallic form is very hard and is a poor electrical conductor at room temperature.
6. Carbon – C:
Symbol C. A non – metallic element belonging to group 14 of the periodic table. Carbon has three main allotropic forms.
*Diamond: occurs naturally and can be produced synthetically. It is extremely hard and has highly refractive crystals.
7. Nitrogen – N:
Symbol N. A colorless gaseous element belonging to group 15 of the periodic table. It occurs in air (about 78% by volume) and us essential constituent of proteins and nucleic acids in living organisms.
Nitrogen is obtained for industrial purposes by fractional distillation of liquid in air. Pure nitrogen can be obtained in the laboratory by heating a metal azide.
8. Oxygen – O:
Symbol O. A colorless odourless gaseous element belonging to group 16 of the periodic table. It is the most abundant element in the earth’s crust and is present in the atmosphere (28% by volume). Atmospheric oxygen is of vital importance for all organisms that carry out aerobic respiration.
9. Fluorine – F:
Symbol F. A poisonous pale yellow gaseous element belonging to group 17 of the periodic table.
The main mineral sources are *fluorite and *cryolite. The element is obtained by electrolysis of a molten mixture of potassium fluoride and hydrogen fluoride.
10. Neon – Ne:
Symbol Ne. A colorless gaseous element belonging to group 18 of the periodic table.
Neon occurs in air (0.0018% by volume)and is obtained by fractional distillation of liquid air.
11. Sodium – Na:
Symbol Na. A soft silvery reactive element belonging to group 1 of the periodic table. Sodium occurs as the chloride in Sea water and in the mineral halite.
12. Magnesium – Mg:
Symbol Mg. A silvery metallic element belonging to group 2 of the periodic table. The element is found in a number of minerals, including magnesite, dolomite and carnallite.
13. Aluminum – Al:
Symbol Al. A silvery white lustrous metallic element belonging to group 3 of the periodic table. The metal itself nus highly reactive but it’s protected by a thin transparent layer of the oxide, which form quickly in air.
14. Silicon – Si:

Symbol Si. Silicon is a metalloid element belonging to group 14 of the periodic table. Silicon is the second most abundant element in the earth’s crust (25.7% by weight ) occuring in various forms of silicon (IV) oxide (e.g *quartz) and in *silicate minerals.
15. Phosphorus – P:
Symbol P. A non – metallic element belonging to group 25 of the periodic table. It occurs in various phosphate rocks, from which it is extracted by heating with carbon (come) and silicon (IV) oxide in an electric furnace (1500°C).
16. Sulphur – S:
Symbol S. A yellow non – metallic element belonging to group 16 of the periodic table. The element occurs in many sulphide and sulphate minerals.
17. Chlorine – Cl:
Atomic Mass Of First 20 Elements With Symbols
Symbol Cl. A halogen element. It is a poisonous greenish-yellow gas and it occurs widely in nature as sodium chloride and in Sea water as halite, carnallite and sylvite.
18. Argon – Ar:
Symbol Ar . A mono atomic Noble gas present in air (0.93%);Argon is separated from liquid air by fractional distillation.
19. Potassium – K:
The Elements, Sorted By Atomic Mass - Angelo State University
Symbol K. A soft silvery metallic element belonging to group 1 of the periodic table. The element occurs in Sea water and in a number of minerals, such as sylvite, carnallite and kainite.
20. Calcium – Ca:
All Elements Atomic Mass
Symbol Ca. A soft grey metallic element belonging to group 2 if the periodic table. The element is extracted by electrolysis of fused calcium chloride and is used as a getter in vaccum systems and a deoxidizer in producing non-ferrous alloys.
Since chemistry is somewhat an abstract subject especially when equations and chemical formula are used, it tends to pose some problem for young chemistry students in understanding how to deal with memorizing elements or cramming their chemical reactions and equations especially during examinations or in practical classes. The above simple methodology of memorizing the first 20 elements of the periodic table is a sure step for a beginner to better acquaint him or herself with the rudiments of chemistry and its numerous applications in metallurgy, manufacturing, medicine and pharmacy.
Atomic Mass Of The First 20 Elements
List of first 50 elements of the periodic table by atomic number including the chemical symbol and the atomic weight. You can print the list of elements by hitting the print button below.
- Atomic Number
| Atomic Number | Chemical Symbol | Element Name | Atomic Weight (u) |
|---|---|---|---|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.002602 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.0121831 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.99840316 |
| 10 | Ne | Neon | 20.1797 |
| 11 | Na | Sodium | 22.98976928 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminium | 26.9815385 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.973762 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.0983 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.955908 |
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.9415 |
| 24 | Cr | Chromium | 51.9961 |
| 25 | Mn | Manganese | 54.938044 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933194 |
| 28 | Ni | Nickel | 58.6934 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.921595 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.4678 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.90584 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.90637 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | 98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.9055 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.8682 |
| 48 | Cd | Cadmium | 112.414 |
| 49 | In | Indium | 114.818 |
| 50 | Sn | Tin | 118.71 |
First 20 Elements Of Periodic Table




